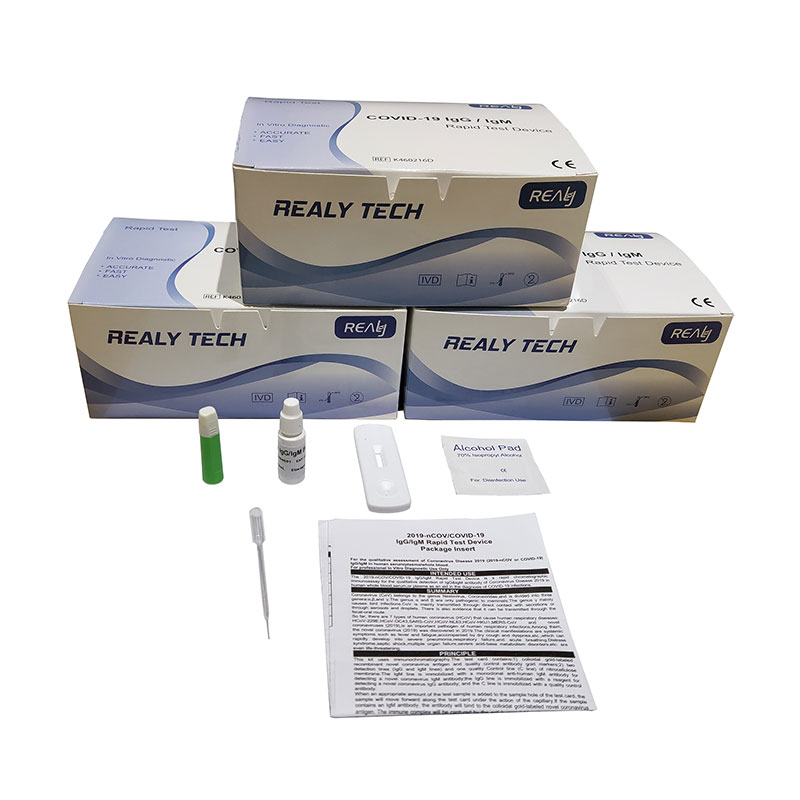
Rapid 2019-nCOV IgG/IgM Test Device for Coronavirus Detection (25 Servings)
Sensitivity and Specificity: The 2019-nCOV/COVID-19 IgG/IgM Rapid Test Device has been compared to a leading commercial RT-PCR testing using clinical specimens.The results show that the 2019-nCoV/COVID-19 IgG/IgM Rapid Test Device has a high sensitivity and specificity.
For IgG testing:
| Method | RT-PCR | Total Results | ||
| 2019-nCOV IgG/IgM Rapid Test Device | Results | Positive | Negative | |
| Positive | 233 | 2 | 235 | |
| Negative | 35 | 287 | 322 | |
| Total Results | 268 | 289 | 557 | |
Relative Sensitivity:233/268=86.94%(95%CI*:82.35%-90.49%)
Relative Specificity:287/289=99.31% (95%CI*:97.52%-99.92%)
Accuracy:520/557=93.36% (95%CI*: 90.96%-95.16%)
*Confidence Interval
For IgM testing
| Method | RT-PCR | Total Results | ||
| 2019-nCOV IgG/IgM Rapid Test Device | Results | Positive | Negative | |
| Positive | 223 | 7 | 230 | |
| Negative | 45 | 282 | 327 | |
| Total Results | 268 | 289 | 557 | |
Relative Sensitivity:223/268=83.21% (95%CI*:78.19%-87.48%)
Relative Specificity:282/289=97.58% (95%CI*: 95.07%-99.02%)
Accuracy: 505/557=90.66% (95%CI*: 87.94%-92.95%)
*Confidence Interval
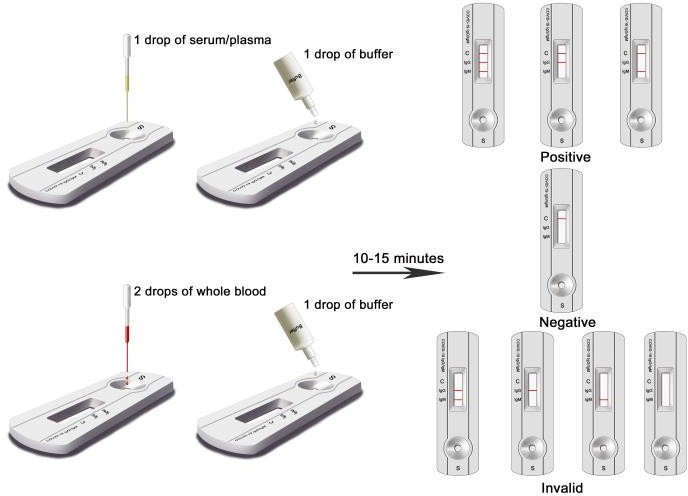
PRINCIPLE: This kit uses immunochromatography.The test card contains:1) colloidal gold-labeled recombinant novel coronavirus antigen and quality control antibody gold markers;2) two detection lines (IgG and IgM lines) and one quality Control line (C line) of nitrocellulose membrane.The IgM line is immobilized with a monoclonal anti-human IgM antibody for detecting a novel coronavirus IgM antibody;the IgG line is immobilized with a reagent for detecting a novel coronavirus IgG antibody; and the C line is immobilized with a quality control antibody.When an appropriate amount of the test sample is added to the sample hole of the test card, the sample will move forward along the test card under the action of the capillary.If the sample contains an IgM antibody, the antibody will bind to the colloidal gold-labeled novel coronavirus antigen. The immune complex will be captured by the anti-human IgM antibody immobilized on the membrane to form a purple-red IgM line,showing that the novel coronavirus IgM antibody is positive.If the sample contains an IgG antibody, the antibody will bind to the colloidal gold-labeled novelcoronavirus antigen,and the immune complex will be captured by the reagent immobilized on the membrane to form a purple-red IgG line,indicating that the novel coronavirus IgG antibody is positive.If the test IgG and IgM lines are not colored,a negative result is displayed.The test card also contains a quality control line C.The fuchsia quality control line C should appear regardless of whether a test line appears.The quality control line is a color band of the quality control antibody immune complex. If the quality control line C does not appear, the test result is invalid, and the sample needs to be tested again with another test card.
Here is what you need to do to conduct the test:
1.Clean finger with alcohol swab.
2.Puncture same finger with finger pricker to get blood sample.
3.To release the needle cover turn clockwise following arrows on the cover.
4.Use the dropper to draw blood to apply to test cassette.
5.Check the markings on the cassette 10mins later to see the result.
Coronavirus (CoV) belongs to the genus Nestovirus, Coronaviridae,and is divided into three genera:α,β,and γ.The genus α and β are only pathogenic to mammals.The genus γ mainly causes bird infections.CoV is mainly transmitted through direct contact with secretions or through aerosols and droplets. There is also evidence that it can be transmitted through the fecal-oral route.
So far, there are 7 types of human coronavirus (HCoV) that cause human respiratory diseases: HCoV-229E,HCoV-OC43,SARS-CoV,HCoV-NL63,HCoV-HKU1,MERS-CoV and novel coronaviruses (2019),is an important pathogen of human respiratory infections.Among them, the novel coronavirus (2019) was discovered in 2019.The clinical manifestations are systemic symptoms such as fever and fatigue,accompanied by dry cough and dyspnea,etc.,which can rapidly develop into severe pneumonia,respiratory failure,and acute breathing.Distress syndrome,septic shock,multiple organ failure,severe acid-base metabolism disorders,etc. are even life-threatening.
The 2019-nCOV/COVID-19 IgG/IgM Rapid Test Device is a rapid chromatographic immunoassay for the qualitative detection of IgG&IgM antibody of Coronavirus Disease 2019 in human whole blood,serum,or plasma as an aid in the diagnosis of COVID-19 infections.